Monday July 30, 2007
This video was referenced last Tuesday when Jiangshui gave his paper presentation. It represents a drop of colored water on a thin triangular polymer film causing a phenomenon called "Capillary Origami." It was taken from "Capillary Origami:Spontaneous Wrapping of a Droplet With an Elastic Sheet" by Charlotte Py, Paul Reverdy, Lionel Doppler, Jose Bico, Benoit Roman, and Charles N. Baroud from Phy.Rev.Lett.98.156103 (issue 13 April 2007).I think it is really cool!
This morning, it is necessary to recalibrate the Tensiometer to water first, as it was used in another application over the weekend. We prepared a new 0.09% surfactant solution, then did three runs to determine its surface tension. The three readings were averaged, and the surface tension of the 0.09% solution is 46.5. Jiangshui now wants us to float films on water with surfactant added. We calculated how much of the 5% surfactant stock solution we needed to add to 200 ml of water to achieve the desired surfactant concentration. We will be counting the wrinkles on films floating on 0.03%, 0.08%, 0.12% and 3% surfactant solutions. Jiangshui's surface tension readings for the various surfactant concentrations are different from our readings.
Tomorrow we will prepare films, float them on the various surfactant concentrations, and count wrinkles!
Monday, July 30, 2007
Posted by
Chaug Biology Research
at
7:52 AM
0
S.Dickson MRSEC Research Summer 2007
![]()
Friday, July 27, 2007
Friday July 27, 2007
Efren and I arrived by 9 to set up the 0.12% solution for three tensiometer readings. The first result is 40, the second run gave us 44, and the third run gave us 42.
Jiangshui then suggested that we determine the surface tension of a 3% sample. This seems to be quite a jump in concentration, but he wants us to see that there is a limit to the effect of surfactant concentration on the surface tension of water. We will investigate both .30% and 3%.
At 10 am we attend the dissertation defense of Edwin P. Chan, a member of Dr. Alfred Crosby's group. This is Dr. Chan's fourth year; upon receipt of his degree today, he will do postdoctoral work at M.I.T.. His dissertation, "Adhesion of Patterned Polymer Interfaces", was inspired by nature; specifically the ability of beetles, bugs, and geckos to climb up walls.
He examined research that indicated that as the mass of the organism increases, the number of setae per area increases while the diameter of each seta decreases. In addition, the shape of the setae vary in nature, some are nearly round (bugs) while others are nearly triangular (gecko). Edwin compares adhesion using a single smooth surface to a surface with a patterned series of posts, then he varies the size, number, and shape of the posts to see the effects of these changes on adhesion. After this work was concluded, he determined that the establishment of these patterns was both labor intensive and expensive, so he sought another means to establish a pattern of adhesion posts. He then investigated wrinkling. He was able to establish a relationship between wrinkling pattern, pattern orientation, and the area being subjected to the stress that causes wrinkling. This provides a quick, inexpensive, and simple way to affect adhesion. This was an extremely interesting presentation, and Dr. Chan's knowledge and ability to explain precisely what he had done, what it meant, and the mathematical and scientific principles behind it on multiple levels was so impressive. I did not feel lost at any time, even though all of the math he presented was most assuredly way over my head. He could rapidly go from gecko feet to Young's modulus and back again, explaining why wrinkling would increase adhesion.
We returned to the lab to set up another reading for the 0.12% solution, then went to lunch. The usual Friday fare was followed by a 4th year PhD candidate's explanation of her work. Liz is a graduate of Carnegie-Mellon with a degree in Chemistry.
She is working on nanoparticles for drug delivery systems, concentrating on the use of gold and PEG (polyethylene glycol) nanoparticles, which are amphiphilic and aggregate at an oil/water or nonpolar/polar liquid interface. We then returned to the lab to complete our work with the tensiometer.
Posted by
Chaug Biology Research
at
10:41 AM
0
S.Dickson MRSEC Research Summer 2007
![]()
Thursday, July 26, 2007
Thursday July 26, 2007
Today the tensiometer is really getting a workout. We are determining the surface tension of the solutions we prepared yesterday, each concentration in triplicate. The 0.03% solution results from yesterday are 59, 60, and 61. We are satisified that this surface tension is around 60.
We then empty the 0.03% solution from the syringe, and rinse the syringe three times using the 0.06% solution. We then set up the tensiometer to take three readings on this solution, and the surface tension of the 0.06% solution was determined to be 50, 51.5, and 51, so the average surface tension is 51 for 0.06% surfactant solution. We will continue with the remaining solutions tomorrow.
Posted by
Chaug Biology Research
at
1:40 PM
0
S.Dickson MRSEC Research Summer 2007
![]()
Wednesday July 25, 2007
As soon as we arrived, we headed for the microscopy lab to receive instruction on the operation of the Atomic Force Microscope (AFM). We will not use this instrument, but Ji Xu explained the principles of its operation and how to use it.
The AFM is not optical; it has a tip (visible only using an optical microscope) that vibrates vertically in response to an oscillating voltage, so that in effect it gently taps the surface of the sample at a constant amplitude as it moves along its surface. The constant amplitude of the tapping allows the tip to move up and down with the variations of the topography of the sample. The tip is attached to a cantilever which is just barely visible to the naked eye; this is attached to a black matrix that is large enough to be manipulated into position on the instrument (below, left). Prior to analyzing the sample, the AFM must be calibrated for that specific sample. First, the tip size is selected; the smaller the tip, the higher the possible magnification. Resolution is determined by the number of oscillations, or vibrations, of the tip on the sample surface, and magnification is again affected by the amplitude of the oscillation of the tip.
A laser beam shines down onto the cantilever and, as the tip moves across the topography of the sample, the beam is reflected at different angles. The reflected laser is collected by receptors that are analyzed and interpreted by the computer to create images. The image on the left of the screens below is the topography of the sample; higher structures appear lighter in color. The images on the right indicate the hardness (how densely the molecules in the sample are packed) of the sample: dark areas are hard and light areas are soft. Ji Xu has hexagons that self-assembled as his polymer annealed. A close up of one of the hexagons is seen below, left, while several are visible in the less magnified image seen below right.
Results are also graphed for interpretation (below, left). The sample can be manipulated under the AFM to a position that correlates to a position previously viewed under a high resolution optical microscope (below, right) so that images obtained from the optical microscope can be compared to the images obtained using the AFM.
After lunch, we prepared surfactant solutions of various concentrations that we will use in next week's wrinkling experiments. From the 0.5% solution prepared on Wednesday, a 1/4 dilution was made, resulting in a 0.1% solution. Twelve ml of the 0.1% solution was mixed with 8 ml of water to prepare a 0.06% solution; 18 ml of the 0.1% solution was diluted with 2 ml of water to create a 0.09% solution. A 0.12% solution was made by mixing 4.8 ml of 0.5% surfactant solution with 15.2 ml of water. We then set up the Tensiometer to measure the surface tension of the 0.03% solution again.
At 2 pm we went with Jaingshui to meet with Dr. Menon of the physics department in Hasbrouck. The first discussion focused on our progress with wrinkling, use of the reflectometer, and data analysis with ImageJ and Origin software. We then discussed problems that we could encounter using the surfactant. The surfactant is amphipathic, and the polar tails actually stick up from the surface of the water, making the environment at the surface of the drop different from the drop's internal environment. The same is true in a bowl of water/surfactant solution: the hydrophobic tails of the surfactant stick up while the hydrophilic heads are oriented toward the water. In the rest of the water, the hydrophobic tails of the surfactant are attracted to one another and form mycellae. The mycellae eventually form spheres (head to head/tail to tail attraction). If the spheres are broken apart (agitation, heat) they may reassemble as cylinders. If the concentration of the surfactant continues to increase, and the cylinders are broken apart, then lamellae may form. The formation of these various structures is dictated by the general rule that material tries to form the geometrical shape with the smallest surface area relative to its concentration.
Jiangshui then discussed the next focus of his research with Menon. Jaingshui will be working to create experimental evidence to support the mathematical explanation for wrinkling patterns.
Posted by
Chaug Biology Research
at
11:17 AM
0
S.Dickson MRSEC Research Summer 2007
![]()
Tuesday July 24, 2007
Today we prepared solutions with various concentrations of surfactant from a 5% stock solution of Dodecylsulfate, Sodium salt, 99% (the surfactant) in water. Jaingshui prepared the stock by dissolving 22.5 g of the salt in 450 ml of water. We prepared a 1/10 dilution of the stock to create a 0.5% solution, then a 1/10 dilution of that to prepare a 0.05% solution. By mixing 18 ml of 0.05% with 12 ml of water, we prepared a 0.03% solution.
We then set up the tensiometer to determine the surface tension of the water/surfactant solution. The purpose of a surfactant is to reduce surface tension. We received a surface tension reading of 61.
After lunch was the Russell group weekly meeting. Dr. Russell set the sequence of presentations, then spoke with individual group members about their progress, expenditures, and references. He spoke with some of the newer PhD candidates about the upcoming cumes. First and second year PhD candidates must take exams (cumes) as part of their program. The exams are offered on the second Saturday of the even numbered months, and consist of six questions; two chemistry, two physics, and two engineering based. PhD students must answer four of the six questions at each session. The students must pass four Cumes in a row, or five total.
Jiangshui was first to present a paper, which was titled "Capillary Origami". A drop of water placed on a nanomembrane causes the nanomembrane to adhere to the surface of the droplet and conform to its shape in a manner that uses the least amount of energy. Be sure to watch the video of the folding process in this website.
The second presentation was by Jodie, and was a synopsis of her PhD dissertation at M.I.T. entitled "Ion Transport-Electrochemical Energy: How Do Ions Transport in a Polymer Electrolyte." Her research focus was trying to improve the generating power of batteries; specifically to develop a medium to sit between the anode and cathode that transported ions like a liquid but has the mechanical properties of a solid. Third to present was Tomomi, who is testing various polymers that may improve the productivity of fuel cells. Currently, Nafion is the main conductor in fuel cells, but it is expensive to produce and does not perform well at high temperatures. As Dr. Woudenberg discussed in his dissertation defense on July 17, this is an area of extensive research and study. There is a great deal going on here at UMass to improve energy production by both fuel cells and photoelectric cells to reduce our dependence on fossil fuels. Jodie also made the final presentaton of the day, discussing piezoelectricity. Again, the idea of generating power without generating pollutants or dependence on foreign production is receiving attention.
Posted by
Chaug Biology Research
at
9:38 AM
0
S.Dickson MRSEC Research Summer 2007
![]()
Monday July 23, 2007
Today we are beginning our work on the effect of varying surface tension on wrinkling. First, we learn to use the Tensiometer. This instrument has a precise set up regimen. After turning the instrument on, a microsyringe is filled with the solution to be analyzed (below left).
Then the syringe is inserted into the syringe holder, just to the right of the thermometer. A cuvette has one ml of water added to it, then it is covered with parafilm. A hole is cut into the parafilm so that the tip of the syringe can be inserted into the covered cuvette; all this is done to prevent evaporation of water from the surface of the drop being analyzed during the measuring period. It is critical that the syringe is precisely perpendicular to the water surface, and that the drop is as large as possible without falling off the tip of the syringe.
As is true of every instrument we have encountered in the lab, the Tensiometer is computer driven. The software, SCA20, is initiated and the parameters for the system and for the ambient conditions are entered into the computer. Once the program is set, the computer directs the volumes to be dispensed from the syringe to create the drop to be analyzed (below, left).
The data is recorded 30 times a minute for an hour. It takes some time for the drop to become stable, so the first ten to fifteen minutes of readings are discarded. The readings then become stable and are displayed alphanumerically as well as graphically (above, right). Each of us set up the instrument and software to measure the surface tension of water. We achieved readings of 70, 71, and 72; the expected surface tension was 72. Pretty good!
At the end of the day, we attended a seminar presented by two professors from Tsinghua University in Beijing, considered China's M.I.T., which has a total enrollment of 30,000 students. Dr. Quingling Feng presented her work on :The Fabrication and Characterization of Scaffolds for Tissue Engineering", which explained the use of biomimetic composites to encourage bone growth. The biomimetric composites are based on studies of natural bone; nanohydroxyapatite/collagen can be molded on PLLA to create a bone scaffold very similar in structure to natural bone. In some work, chitin was used instead of hydroxyapatite in conjunction with the collagen, and more crosslinking between molecules (therefore higher compression strength) was found. A combination of the two polymers was used to repair fractures in goat tibia, and the healing time was decreased. The material has received approval for trials in humans in China.
The second presentation was by Professor Guosheng Gai, whose field of expertise is the behavior of fine powders. His current research investigates the construction of composite particles and modification of their shape. He controls the microstructure within the material to affect particle shape. He has been able to create conductive plastics through particle coating. This was hard for me to follow.
Posted by
Chaug Biology Research
at
8:05 AM
0
S.Dickson MRSEC Research Summer 2007
![]()
Wednesday, July 25, 2007
Friday July 20, 2007
Efren and I arrived at Conte by 7:30. He set up the X-Ray Reflectometer while I spin coated some films.
We then placed one of my specimens in the reflectometer to measure the thickness. We both caught up on our blogging during the 90 minute processing period. The data was just OK...we only got three consistent waves in a row, and there should be five in a row to get a good measurement of film thickness. We calculated the thickness to be about 100 nm thick, which is thicker than either Jaingshui or Dave get ( about 86 nm). The second slide was processed, but this time we got NO data...I put the specimen in backwards, so there was no film on the slide, just glass. Sheesh! There seem to be an infinite number of ways to mess up.
The normal Friday noon lunch for R.E.T.s (us) and R.E.U.s (undergrads doing research) was held, followed by two presentations by doctoral candidates. Kate was first to present; she received her undergrad degree at RPI and is working with the Lesser group on improving the environmental resistance of PBO. PBO replaced Kevlar as the primary constituent of bullet-proof gear because it has a higher tensile strength than Kevlar and is lighter in weight and simpler and less expensive to produce. It has failed, however, after prolonged exposure to UV light and moisture. The project is hoping to develop a polymer to coat the PBO that will confer UV and moisture resistance to the substance without reducing tensile strength.
The second presentation, by Simon, was entitled "Novel Hybrid Polymers Incorporating Carboranes as Pendant Groups". Simon is a fourth year PhD student approaching the end of his work. A carborane is an icosohedral cluster with Boron and Carbon integrated in the structure.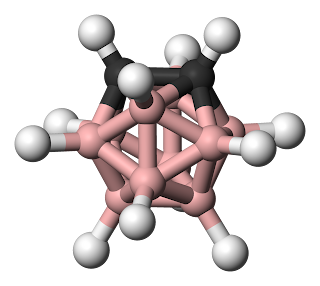
Posted by
Chaug Biology Research
at
1:31 PM
0
S.Dickson MRSEC Research Summer 2007
![]()
Thursday July 19 2007
We are trying to perfect our wrinkling techniques. This field of study is procedurally intensive and requires much practice before we can generate useful data. Because we are working on a nanoscale, the slightest imprecision in drop delivery or film preparation is magnified in our calculations. We each make three films, then float and wrinkle them, recording our results with photographs. The images are downloaded from the digital camera onto the computer, then we upload them onto our jump drives and bring them back to the Conte computer lab for measurement and calculations using Image J and Origin software packages. We spend quite a bit of time discussing how we could reproduce this experience in the classroom. Saran Wrap or any commercially available "membrane" is too thick to form wrinkles. We finally decided the closest we could come would be to wrinkle the film that forms on pudding as it sits in the refrigerator.
Posted by
Chaug Biology Research
at
10:41 AM
0
S.Dickson MRSEC Research Summer 2007
![]()
Wednesday July 18, 2007
During the morning, we had more practice making films and wrinkling. We made two films each, then floated and wrinkled them, taking pictures to use for data analysis later. After lunch, Dave made a film and we placed it in the reflectometer to determine its thickness. While it was processing (90 minutes) we made some films on silicon wafers instead of glass slides.
We lowered the spin speed to thicken the polystyrene coat. We then met Ling who showed us a new instrument for measuring thickness, the interferometer. The interferometer is made by Filmetrics and uses optics to measure thicknesses between 30 nm and 1 micron. It is not as accurate as the X-Ray reflectometer. More information about the interferometer can be found here.
Posted by
Chaug Biology Research
at
10:29 AM
0
S.Dickson MRSEC Research Summer 2007
![]()
Friday, July 20, 2007
Tuesday, July 17, 2007
We attended the PhD dissertation defense of R.C.Woudenberg, entitled "Anhydrous Proton Conducting Materials for Use in High Temperature Polymer Electrolyte Fuel Cells". His research proposed improvements to expand the optimal operating temperatures of polymer electrolyte membrane fuel cells (PEMFCs) from their current range of 80 degrees C to 120-200 degrees C. PEMFCs have high efficiency and are non-polluting, but current examples have a low operating temperature. This research proposed the synthesis of a copolymer, joining a molecule that performs well at low temperatures with a molecule that performs well at high temperatures; this also correlates with operation under hydrous and anhydrous conditions.
After the presentation, we transferred images of wrinkling experiments to the computers in the Conte lab and did some data reduction using Image J and Omega. I spent some time writing out and fine tuning a procedure for operation of the X-Ray reflectometer. The finished procedure is available to you in the column to the right under "Procedures in the Lab".
Every Tuesday at 1:30, all members of Dr. Russell's research team meet to share information and gain ideas from one another. There are about 30 people in attendance, and we are welcomed as guests. Each week, two group members give major presentations of about 30 to 45 minutes each that provide in-depth information about the progress of their research. Occasionally, Dr. Russell will invite someone from another group (operating on another floor of Conte with a different specialty) to present their findings. In addition, Dr. Russell will ask two or three individual group members to read and present a synopsis of a journal article he has read or that they themselves have found particularly interesting and relevant to their research. The purpose of the meeting is not only to keep everyone informed about what is going on within the group, but also to help the researchers be certain that they aren't missing something. Everyone works together and provide support to one another. I am infinitely impressed by the amount and depth of knowledge Dr. Russell possesses.
This week, journal article presentations include "Differential Interference Contrast Microscopy" followed by "A Menagerie of Interface Substructures in Copolymer Systems". Maisie then presented her post-doc work on "Virus Nanoparticles on Patterned Wafers". The goal of her research is to create a perfect pattern of virus nanoparticles on a surface so that there is a perfect distribution of feature size, and a perfect center to center distance between the features, and no line edge roughness. She is using the Cow Pea Mosaic Virus because every one of them is the same size (called monodispersal). If she is able to accomplish this, it would be very useful in data storage. The second presentation discusses "Modification of Horse Spleen Ferritin". Most of this one is way over my head and not especially interesting to me. Juangshui presents the last paper of the day concerning wrinkling. The meeting lasts for three hours with a short ten minute break part way through. It is not always easy to stay focused.
Posted by
Chaug Biology Research
at
8:50 AM
0
S.Dickson MRSEC Research Summer 2007
![]()
Monday July 16, 2007
Practice, practice practice. We are making three films each at a time. One of us will make four films and load the fourth into the X-Ray Reflectometer to determine the thickness.
If you would like to get an idea of how complicated the reflectometer is to use, reference the reflectometer procedure to the right. The adjustments to focus the beam take a great deal of time.
We floated and dropped and measured and calculated for hours. We also met with Dr. Russell, our supervising professor, to inform him of our accomplishments so far. Efren and I provided him with our blog addresses, and Dave explained the procedures we have used. Dr. Russell explained to us that the principle of the X-Ray reflectometer was the same as the principle we encounter when we look at an oil slick on water and see the colors - the white light gets broken up into different wavelengths as it passes through the oil and back out again. The X-Rays get redirected as they pass through the sample and emerge at different angles.
Posted by
Chaug Biology Research
at
8:20 AM
0
S.Dickson MRSEC Research Summer 2007
![]()
Wednesday, July 18, 2007
Scanning Electron Microscope, Dr. Hashimoto
Friday July 13, 2007
Dian took us into the electron microscope room to use the scanning electron microscope and to see the transmission electron microscope. Dian had prepared her nanotubes the day before. The nanotubes self assemble when the temperatures they are exposed to vary (this sounds easy and rather sloppy, but like everything that is done here, it is neither!). She placed her sample on a metal sample holder (looks like a circular, thick brass plate), adhering it with double sided carbon tape , chosen because it can conduct. We take the mounted sample into another room, where the sample is spatter coated with a layer of gold between 1 and 2 nanometers thick.
The sample is then brought back to the Electron Microscopy Lab and it is loaded onto the SEM sample insertion device.
The picture immediately above on the right shows the sample inside the SEM sample chamber. At the time of this photo, Dian has evacuated the SEM sample chamber, and the beam has been centered. Control knobs are used to move the sample horizontally and vertically within the chamber. The contrast and magnification knobs are used to obtain the sharpest possible image at the desired magnification. We located tubes at a 40,000 magnification. The image below left is a low magnification of the tube sample, and to the right is a high magnification showing tubes.
All of us had an opportunity to move the sample, adjust the magnification, and take pictures. These pictures are digital, so they are easily transferable as files. The TEM is quite old however, and all of the pictures are taken on photographic film and must be chemically developed (old school indeed). Using the SEM, Dian is trying to see how smooth the surfaces of the tubes are.

Posted by
Chaug Biology Research
at
11:49 AM
0
S.Dickson MRSEC Research Summer 2007
![]()

